
Dynamic Data Analysis – v5.12.01 - © KAPPA 1988-2017
Chapte
r 2 – T heory- p52/743
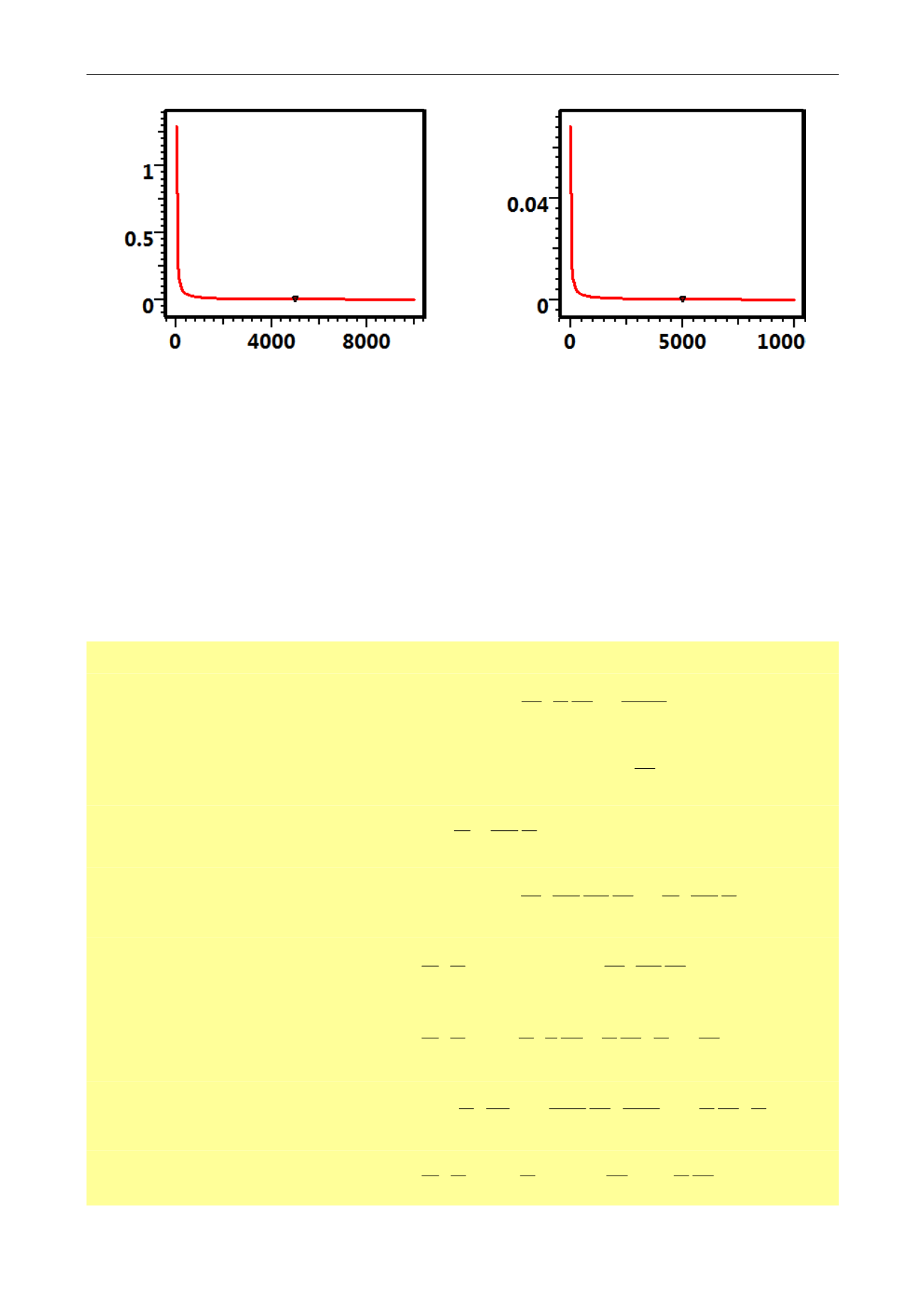
Fig. 2.I.3 – B
g
[SCF/RCF] vs p [psia]
Fig. 2.I.4 – c
g
[psi
-1
] vs p [psia]
We could just use these PVT elements straight into a numerical simulator. However we would
be short of diagnostic plots if we were just blindly simulating and matching.
The idea is to rearrange the equations by changing variables in a gas diffusion equation that
looks like the slightly compressible fluid equation. If we do so we could extend the
methodology and diagnostic plots developed for the linear process to the nonlinear process of
gas diffusion. This is done by introducing pseudopressures.
2.I.2
Derivation of the real dry gas diffusion
We deviate from our initial derivation just before the slightly compressible fluid assumption:
Raw diffusion equation:
t
x
p
x
k
x
0002637
.0
The real gas law gives:
ZnRT
PV
where
M
m
n
This gives the gas density:
Z
p
RT
M
V
m
Diffusion equation becomes:
Z
p
RT
M
t
x
p
Z
p
RT
M
x
k
x
0002637
.0
Which simplifies to:
x
p
Z
p
x
k
Z
p
t
x
0002637
.0
The first term develops as:
t
p
Z
p
pp
Z
p Z
p
Z
p
t
T
1
The gas compressibility is written:
T
T
T
g
Z
p
pp
Z
RTZ
Mp
p Mp
RTZ
p
c
1
So:
t
p
Z
p
c
t
p
c c
Z
p
Z
p
t
t
g
f
















